Two groups of researchers at the University of Macau (UM) and Shenzhen Institute of Advanced Technology (SIAT) have developed a new drugs associated with novel drug delivery system to treat Parkinson’s disease (PD). The UM group is led by Centre for Cognitive and Brain Sciences (CCBS) Professor Yuan Zhen, who is also the head of CCBS, and the other group is led by Prof. Zonghai Sheng of SIAT. This work was published in the Research (Science sister journal; IF: 11.036). Jing Liu and Duyang Gao as Prof. Zhen Yuan’s current and previous PhD students are the 1st author of this paper.
PD, a neurodegenerative encephalopathy, seriously endangers human health. The clinical treatments for PD mainly contain chemical drugs (e.g., dopaminergic-type drugs, anticholinergics, or glutamate antagonist) through oral administration or intravenous administration. However, the blood–brain barrier (BBB) greatly decreases the efficiency of drugs entering the brain and reduces their therapeutic effect. By contrast, meningeal lymphatic vessels (MLVs) have been considered an important drainage system to maintain intracranial fluid balance and immune monitoring. Very recently, nanomedicine has been delivered into the brain via MLV route for glioma therapy, confirming the feasibility of this delivery route. However, the abundant immune cells in the MLV route can endocytose the nanomedicine, thereby dramatically reducing the drug transport efficiency. In addition, the low targeting ability of these nanomedicines reduces their therapeutic efficacy with increasing side effects.
To this regard, we reported an MLV delivery strategy for targeted therapy of PD using natural killer (NK) cell membrane-camouflaged curcumin liposomes (BLIPO-CUR) (Fig. 1). The biomimetic liposomes spread into the MLVs from cervical lymph nodes after subcutaneous injection in the neck site, escaping from the phagocytosis of macrophages and specifically recognizing the damaged neurons. Besides that, BLIPO-CUR could efficiently clear reactive oxygen species (ROS) and absorb α-synuclein (α-syn) species owning to the expression of Toll-like receptor 4 (TLR4) on NK cell surface. Importantly, BLIPO-CUR could inhibit the spread of α-syn between neurons and glial cells as well as astrocytes and microglials, leading to enhanced PD therapeutic efficiency of curcumin. Our results hold great promise for the delivery of biomimetic liposome nanomedicines for efficient therapy of neurodegenerative encephalopathy.
We discovered that the membrane incorporation enables BLIPO-CUR to target the damaged neurons, thus improving their therapeutic efficacy through clearing reactive oxygen species, suppressing the aggregation of α-synuclein, and inhibiting the spread of excess α-synuclein species. Compared with the conventional intravenous injection, this MLV administration can enhance the delivered efficiency of curcumin into the brain by ~20 folds. The MLV route administration of BLIPO-CUR enhances the treatment efficacy of Parkinson’s disease by improving their movement disorders and reversing neuron death. Our findings highlight the great potential of MLV route administration used as targeted delivery of drugs to the brain, holding a great promise for neurodegenerative disease therapy.
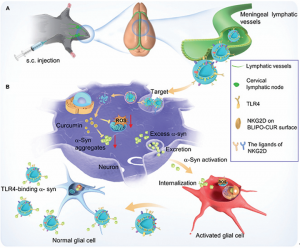
Fig. 1. Schematic illustration of BLIPO-CUR delivered through MLVs and enhanced therapy for PD. (A) Subcutaneous (s.c.) administration of BLIPO-CUR at the neck of mice. (B) After crossing the MLVs, BLIPO-CUR could target the broken dopaminergic neurons through the integrations of the NKG2D receptor and its ligands. After the internalization of BLIPO-CUR, curcumin could clear the ROS and inhibit the α-syn to aggregate. The extracellular BLIPO-CUR could segregate the excess α-syn species among neurons and glial cells by TLR4 binding with α-syn.
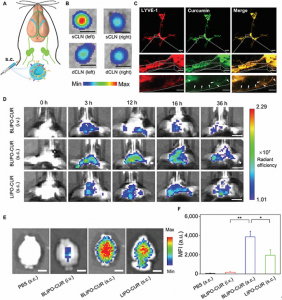
Fig. 2. In vivo fluorescence imaging of BLIPO-CUR drained through MLVs in healthy mice. (A) Illustration of MLV route-mediated delivery of BLIPO-CUR into brain tissues. (B) The representative ex vivo fluorescence images of CLNs of the BLIPO-CUR (subcutaneously) group. sCLN, superficial cervical lymph node. Scale bars, 1 mm. (C) Representative images of MLVs labeled by Lyve-1 (red) and curcumin (green) in mice treated with subcutaneous injection of BLIPO-CUR. Scale bars, 1 mm (top), 200 μm (middle), and 100 μm (bottom). (D) Real-time fluorescence images of the mice before and after intravenous (i.v.) injection of BLIPO-CUR, subcutaneous injection of BLIPO-CUR, and subcutaneous injection of LIPO-CUR. Scale bars, 5 mm. (E) Representative in vivo imaging system (IVIS) images of the brains of the mice ex vivo at 12 h after injections. Scale bars, 5 mm. (F) Semiquantitative results of the brain of mice ex vivo in (E). All data are shown as means ± SD; *P < 0.05; **P < 0.01; one-way ANOVA.
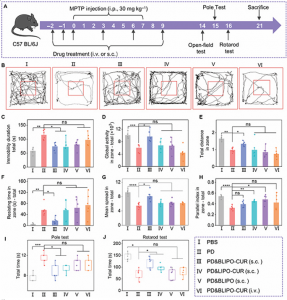
Fig. 3. Protective effects of BLIPO-CUR delivered through MLVs on MPTP-induced mouse models of PD. (A) Schematic illustration of the schedule for PD mouse model establishment and drug treatments. i.p., intraperitoneal. (B) Open-field test. Representative track test sheets. (C to H) Parameter analysis shows the change in global activity, resting time, total distance, mean speed, duration of immobility, and parallel index in the open-field test. (I) Pole test. The average time of mice to climb the whole pole equipment. (J) Rotarod test. The averaged holding times of mice on the rotarod. All data are shown as means ± SD; n = 6 animals for each group. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; one-way ANOVA. ns, not significant.

