Recently, the research team led by Dr. Haiyan Wu at the Centre for Cognitive and Brain Sciences (CCBS), University of Macau, published a new study titled “An intracranial dissection of human escape circuits” in Nature Communications. This study utilized intracranial electrophysiological signals to explore the neural mechanisms underlying human escape behavior. The findings revealed that the cognitive fear circuit encodes the level of perceived threat during the information processing stage, while the reactive fear circuit is predominantly activated during the actual execution of escape behavior.
Unresolved Questions from Previous Research
Escape is a critical defensive behavior exhibited by both animals and humans when confronted with threats. This behavior involves distinct stages: after detecting a predator, the prey must first perceive and process threat-related information (information processing stage) and then determine the optimal timing for escape (escape stage). While previous studies using fMRI and EEG have investigated the neural mechanisms of escape behavior in humans and animals, key questions remain unresolved. Research has identified two fear circuits involved in escape: the reactive fear circuit and the cognitive fear circuit. However, the specific roles of these circuits in different stages of escape remain poorly understood. Moreover, the interaction between the reactive and cognitive fear circuits has yet to be explored. To address these gaps, Dr. Haiyan Wu’s team recorded intracranial EEG (iEEG) data from epilepsy patients performing an escape task, examining the contributions of these neural circuits across different escape stages (Figure 1).
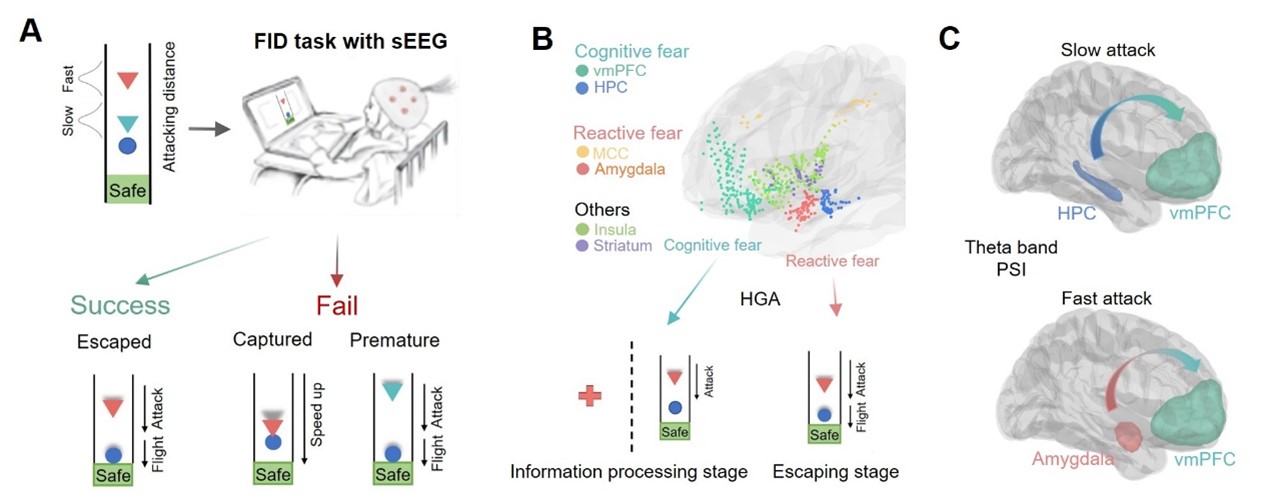
Figure 1. Summary of the current study. (A) Experimental paradigm. (B) The reactive and cognitive fear circuits are primarily engaged during threat information processing and escape execution, respectively. (C) Phase slope index (PSI) analysis revealed theta-band information flow from the hippocampus (HPC) to the ventromedial prefrontal cortex (vmPFC) under slow attacks and from the amygdala to the vmPFC during fast attacks.
Simulated Escape Task Mimicking Real-World Scenarios
The study employed a modified flight initiation distance (FID) task. In this paradigm, participants were instructed to imagine themselves as prey and escape from a virtual predator. Two types of predators with varying urgency levels were introduced: fast-attacking and slow-attacking. Fast-attacking predators initiated attacks earlier, requiring participants to make rapid escape decisions, whereas slow-attacking predators delayed their attacks, allowing more time and a larger buffer zone for escape (Figure 2). High-gamma band activity (HGA) from six brain regions was analyzed to assess local neuronal population activity during the escape process. By comparing neural responses to different threat types, the study elucidated how the human brain processes varying levels of threat.

Figure 2. Experimental paradigm of the current study.
Intracranial Electrophysiology: A High Spatiotemporal Resolution Approach
While functional magnetic resonance imaging (fMRI) has provided valuable insights into neural activity during human escape behavior, its low temporal resolution limits the ability to capture rapid neural dynamics. Conversely, scalp EEG offers high temporal resolution but suffers from poor spatial resolution and signal-to-noise ratio. iEEG overcomes these limitations by combining millisecond-level temporal precision with precise spatial targeting of deep brain structures, offering a comprehensive view of neural dynamics during escape (Figure 3). Crucially, iEEG signals span multiple frequency bands, each encoding distinct aspects of neural communication. High gamma activity (HGA), a reliable proxy for local neuronal population activation, enables fine-grained analysis of neural activity.
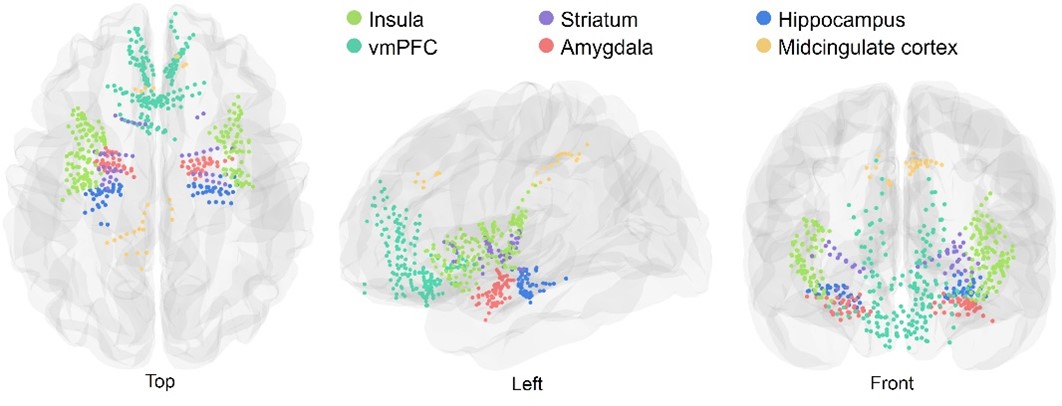
Figure 3. Anatomical locations of iEEG electrodes.
Key Findings
At the behavioral level, the study found that participants chose to escape earlier under fast-attacking conditions but delayed their escape under slow-attacking conditions. At the neural level, regions within the cognitive fear circuit, including the vmPFC and hippocampus, encoded threat levels during the information processing stage. In contrast, the reactive fear circuit (encompassing the midcingulate cortex and amygdala) was prominently activated during the actual escape stage, particularly under rapid attacks (Figure 4). Notably, under fast-attacking conditions, significant theta-band information flow was observed from the amygdala to the vmPFC, suggesting dynamic communication between the reactive and cognitive fear circuits. This interaction may facilitate rapid responses to imminent threats, which are critical for survival. Together, these findings illuminate the distinct yet complementary roles of the reactive and cognitive fear circuits in promoting successful escape behavior.

Figure 4. The cognitive fear circuit encodes threat levels during information processing, while the reactive fear circuit is significantly activated during escape execution.
Paper Information: Zhang, H., Cheng, J., Hu, K. et al. An intracranial dissection of human escape circuits. Nat Commun 16, 5520 (2025). https://doi.org/10.1038/s41467-025-60666-9

